Abstract
Chronic myeloid leukemia (CML) needs taking life-long tyrosine kinase inhibitor (TKI) which arises serious toxicities such as peripheral occlusive disease and pulmonary hypertension as well as low-grade toxicities. Treatment-free remission (TFR) can make almost half CML patients achieving deep molecular response (DMR) free from TKIs. We investigated total costs of each TKI considering TFR in Korea.
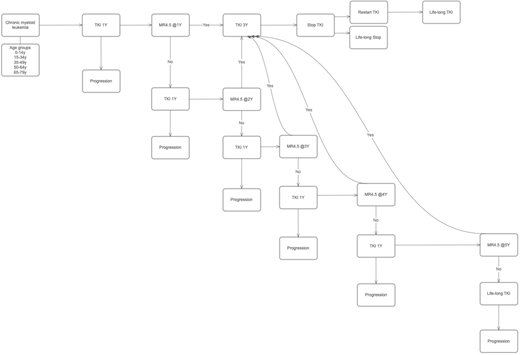
Total numbers of annual newly-diagnosed CML patients were adopted from national cancer registration database. TFR was defined as achieving MR4.5 and lasting for 3 years. The proportion of MR4.5 was adopted from phase III studies of imatinib (IM), nilotinib (NIL) and dasatinib (DAS). TKI management followed life-long Markov model (Fig 1). Willingness to pay (WTP)was calculated for beneficial effects of patients who have achieved TFR and taking no TKIs as 2 times of gross domestic product per capita. Cost of progression was calculated based on the study by Jabbour et al. Patients older than 79y was not included in this study because TFR benefit is not considered in this age group. Duration of treatment and TFR was calculated separately according to age groups. We assumed that taking KTI lasted life-long until median life expectancy in Korea (80.87 years).
Newly diagnosed annual CML patients were 443; 9 in age 0-14, 96 in age 15-34, 125 in age 35-49, 124 in age 50-64 and 89 in age 65-79. The theoretical number of patients who have sustainable TFR was 73 in IM, 93 in DAS and 119 in NIL. TFR as person-year in IM, DAS and NIL were 1940, 2521 and 3247, respectively. TFR (person-year) was very different according to age groups because of different incidence rates; 99.9 in age 0-14, 788.7 in age 15-34, 666.1 in age 35-49, 353.8 in age 50-64 and 33.7 in age 65-79 when IM was applied. TFR (person-year) were 128.2 in age 0-14, 1014.5 in age 15-34, 861.6 in age 35-49, 464.1 in age 50-64 and 52.8 in age 65-79 when DAS was applied. TFR (person-year) were 164.9 in age 0-14, 1305.6 in age 15-34, 1109.4 in age 35-49, 598.3 in age 50-64 and 69.0 in age 65-79 when NIL was applied. WTP according to TFR was 121.3 in IM, 157.5 in DAS and 202.8 billion KW in NIL. Costs by progression were 32.8 in IM, 26.8 in DAS and 22.3 billion KW in NIL. Life-long maintenance costs for patients who did not achieved sustainable TFR without progression were 128.7 in IM, 294.2 in DAS and 327.1 billion KW in NIL. Net costs considering TFR and progression were 40.2 in IM, 163.5 in DAS and 146.6 billion KW in NIL.
Nilotinib was superior to other TKIs in terms of TFR benefit and progression cost according to our life-time Markov model. However, the net cost was lowest in IM. This analysis is limited by our assumption that MR4.5 is achieved by first 5 year and there is no further additional achievement of MR4.5 because of limitation of MR4.5 rates in each TKI trial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal